Working with lye can be intimidating, especially if you don’t know what it is or how to work with it. What, exactly, is lye? And, is it possible to make soap without it?

Over the years, I’ve been asked one question so often now that I figured it was time to write a whole post about it. Even most people who know what lye is, don’t really understand it fully and have a lot more questions about it. They are also concerned about the safety of using it to make soap.
What is Lye?
Lye is a very-alkaline product that is used to make soap, but it is also used in the food and cleaning industries. There are several types of lye, the most known and most commonly used being NaOH, sodium hydroxide, and KOH, potassium hydroxide. All are metal hydroxides and are very basic (alkaline), meaning they have a very high pH. That makes them very caustic, meaning they can give you a chemical burn if you touch them with unprotected skin.
How is lye formed?
Traditionally, lye was made from wood ashes! By allowing the wood ashes to seep in water, a lye-type (or at least a caustic basic) solution was formed. This lye solution is also known as potash, or “pot ash” (from soaking ashes in a pot). In fact, the name potassium comes from “pot ash” since that’s where it was first isolated.
Potash really has more potassium carbonate than KOH, though, which may explain why you can make a solid soap with it despite the fact that KOH is normally used to make liquid soaps. (I’m saying this without having looked into the chemical reaction that takes place, but I’d love to hear somebody else’s take on it.)

Making soap with a wood ash solution (Potash)
Early soap makers used this wood ash solution to make soap by reacting it with whatever oils and fats they had on hand.
I actually did try this myself once, as an experiment. I soaked some of our leftover wood ashes from the fireplace for several days in water. (This works best if you use ashes from hard woods.) I filtered out the ashes and used the solution to make a soap, of sorts, by cooking it over the stove with some filtered, previously-used-for-frying cooking oils, likely olive oil or coconut oil. (I didn’t want to waste good fats on an experiment that I was almost sure I’d fail at.) 😉
While I wouldn’t suggest doing this as a way to make a nice bar of soap, I did find it interesting that I could make it work. My soap doesn’t have the same cleaning power or lather as most soaps I make, but it does resemble soap, and it does have some cleaning ability. I would like to note that mine was a bit gelatinous at first, and it took months of drying it to harden into bars of soap. (I almost gave up and threw it all out.) That could be due to the fact that my ashes were comprised of a mix of many types of wood, with a majority likely being softer woods like pine. It is said that soft woods don’t have the amount of potassium in them needed for making lye for soap. Of course, it may also be due to the type of oil I used.
I’m definitely no expert at making rudimentary pioneer soaps. (Or whatever you want to call them!)

Modern Lye
Lye, at least the sodium hydroxide (NaOH) type, the type normally used for making bar soap, is now made by breaking down a salt water solution (NaCl and H2O) into NaOH, H2, and Cl2 with a membrane cell chloralkali process. (You can read more about the chloralkali process here.)
Making soap nowadays is much easier and much more precise. You can easily buy lye in beads or flakes. Because their purity is known, you no longer have to guess how strong your lye is and how much you will need to make soap. That allows you to make a very accurate recipe that can be repeatedly made in exactly the same way each time. You can also tailor that recipe to use more or less lye depending on how cleansing or how conditioning you want your soap to be. I’ll go into that in more detail some other day, but that’s in reference to “superfatting” a soap or not (using more oil than the amount needed for reacting precisely with the lye to make soap).

Can You Make Soap Without Lye?
The answer to this question really depends on your definition of soap.
What most of us know as real, pure, natural soap, though, can NOT be made without lye!
Some people will argue that they’ve seen soaps without lye in the ingredients, but all of the cases that I have seen are due to one of two scenarios…
Detergent bars (Syndet Bars) of “Soap”
There are many types of bar “soap” on the market that aren’t actually really soap.
While that may sound crazy at first, perhaps some of you will remember some retro Dove commercials where they boasted that Dove wasn’t really soap, but was instead much, much better than soap because it’s less drying.
Here’s one of those fun blasts from the past…
The case of Dove is a bit unusual, though, and I’ll get to that in a minute.
That said, there are many types of solid cleansing products that are sold in bars, like soap, and that are not made with lye. These bars of “soap” are commonly referred to as Syndet bars, which comes from “synthetic detergent” bars. These detergent-based bars are made from a variety of surfactants and not from the chemical process of reacting lye with fat. There are some more natural surfactants, so that doesn’t necessarily make them bad, but it’s important to make the distinction.
I can get into a discussion about which is best some other day, but for now, let’s stick to the facts and just say that syndet bars aren’t really “soap” even though they are often referred to as such. Their pH is generally lower than soap, which may be great for many people, but that doesn’t necessarily make them best for everybody.
Soaps made with “Sodium tallowate,” “Sodium cocoate,” or similar compounds
I’ve had people brag to me that they are using a soap without lye because their soap is made with ingredients like sodium tallowate or sodium cocoate. In fact, even the Dove bars that we mentioned above have those, along with sodium palmitate, in their ingredient list.
What, exactly, are these ingredients?
Well, they are soap! You don’t see lye in the ingredients because the lye has already been reacted with fat to make a soap.
In the case of sodium tallowate, you are looking at a tallow based soap. Sodium cocoate, on the other hand, is a pure coconut oil soap like the laundry soap I’ve showed you how to make on the blog. Sodium olivate, is a pure Castile soap made with olive oil. Yes, I also have a recipe for that!
Going back to the Dove bar we were using as a reference. They say it isn’t soap because the main ingredient is sodium cocoyl isethionate, a mild, non-soap surfactant. On the other hand, their “non-soap” actually does have some soap in it (sodium tallowate, sodium cocoate, and sodium palmitate).
Again, it’s all about semantics, I guess.

Melt and Pour soaps
Sometimes, people will use melt-and-pour soaps to avoid using something with lye in it.
If you are choosing to use a melt-and-pour soap because you, yourself, are afraid of handling the lye yourself, then go for it! I understand people being afraid of handling lye when they’ve never done it before.
If, on the other hand, you are choosing to use a melt-and-pour soap to avoid making something with “lye in it,” then know that your melt-and-soap bar falls into one of the two categories I was talking about above.
Either you are buying a product in which the lye was already reacted with fats to make a soap-based product, or you are using a syndet bar which isn’t really soap. (Or, you are using a mixed product that falls into both of those categories like the Dove bar.)
I don’t think I’ve ever used a melt-and-pour soap myself, but I do get the attraction of wanting something that is very simple to make. (It’s kind of like making a cake from a box mix. You can use it as is, or add things to it to make it your own.) It’s also probably the best way to make a fun project with young kids.
Use a melt-and-pour soap for one of those reasons, though, and not because you want to make soap without lye. Most melt-and-pour soaps will have other chemicals added to the soap (if they are, indeed, soap) to make them meltable (one example being propylene glycol). Natural soaps don’t normally melt into a nice, smooth thick liquid on their own. (Yes, you can melt most soaps down to rebatch them, but they normally turn into a more “rustic” looking soap that isn’t super smooth like m&p soaps are.)
So, in your quest to avoid lye, you may be using something with more questionable ingredients than pure soap.
That brings us to another important question…

Does soap have lye in it?
Not really!
Wait a second… I thought you just said that you needed lye to make real soap!
While you do need lye to make soap, no lye is left in the finished product. (When done correctly, of course.)
Making soap isn’t like baking a cake.
While you are using a recipe, and mixing oils with other ingredients, and mixing them with a blender, and often times pouring them into loaf pans and such, we’re really doing more of a science experiment and making a chemical reaction.
OK, I guess in the process of baking a cake, you are probably doing all sorts of chemical reactions too, but I want you to think of this in a slightly different way…
In the case of baking a cake, you think…
“I’m intolerant to dairy, so I better not put milk in my cake.” or
“I have issues with gluten, so I’d better not use wheat flour.”
In the case of making soap, it’s not quite so simple…
Soap as a Type of Salt
When making soap, you are making a type of salt, a sodium salt of fatty acids. While it’s not like the salt that we use for cooking, that more-familiar type of salt will help us better understand what we are working with anyway.
Table salt is also known as NaCl or sodium chloride. Most people don’t think of salt as being toxic, and they see it as something that is quite natural, right?
But, you wouldn’t want to eat sodium, would you? Or Chloride?
Both sodium and chlorine are quite reactive on their own, and we wouldn’t really want to be handling either one of them, at least not without being very careful. Yet, we all take in some NaCl on a regular basis. In making salt, the reactivity of each element is neutralized, and you end up with a safe product that behaves nothing like its components.
Making soap is very similar. Lye is used to make soap, but your final product doesn’t behave like lye at all. (You also won’t normally see lye listed as one of the ingredients in soap, just as you’ll see “salt” in a food’s ingredient list rather than sodium and chloride.)

The Toxicity of Lye
So, even though we’ve established that your homemade soap doesn’t share the same properties as the lye used to make it, you may still be wondering about the toxicity of lye itself. I mean, if you want to make soap, you are going to have to work with it, right?
Safety precautions when working with lye
I don’t want to belittle the fact that you DO have to be careful when working with lye because it is very caustic! You can get severe chemical burns from handling it and can do other serious damage to other organs. That’s why you should always wear gloves and safety goggles when working with lye. You should also avoid breathing in the fumes that are given off when you mix the lye flakes or beads with water or other liquids.
There are normally around 5 to 10 minutes of making soap where you should be especially careful. This includes the time in which you are mixing the lye with water until you mix it together with your oils.
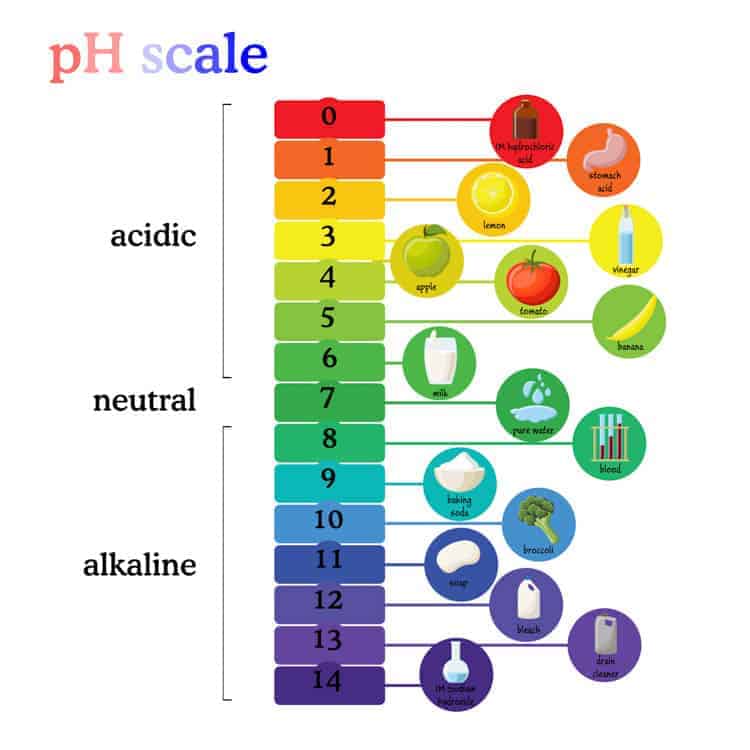
Is lye really toxic, though?
Most of us are familiar with the thought of getting burned by a very strong acid. Some acids at full strength would burn us, yet when diluted enough, we could probably drink them.
Before I continue, let me precede this with a “Please, for goodness’ sake, don’t even consider drinking lye!” 😉
Strong bases behave very similarly to strong acids in that way, meaning that they can burn us in the very same way.
Just because they are highly caustic, though, doesn’t necessarily mean that they are toxic.
You may have seen food grade sodium hydroxide for sale and wondered what that was all about.
Well, you can actually use lye to make certain food products. It is used to cure many types of olives (not my salt-cured olives, though). It is also often used to give some pretzels and some bagels their characteristic outer texture.
It’s not really toxic, but you do have to be very careful when using it!

Do you use separate equipment (a different blender and bowl) for making soap?
For the above reasons, despite how strongly many people feel about the importance of it, I do NOT use separate utensils for making soap.
Some people will argue that you may have the tiniest bit of lye leftover in some little crack of whatever it is you are using to make soap. My answer to that, I guess, is that not only is that unlikely, but, even if you do, “So, what?!?!?”
Lye isn’t really toxic, and I just don’t see the purpose of needing to use separate equipment. I, instead, carefully clean it, which is, of course, very easy to do because you’re essentially just cleaning soap off most of your equipment.
By now, I actually do have a dedicated immersion blender for making soap, but it isn’t because I’m afraid that the tiniest bit of lye may be left in some nook or cranny of it. Instead, it’s more due to the fact that I’m pretty hard on my soap-making blender. So, rather than risk burning out my favorite kitchen one, I use an older blender that my mother-in-law was getting rid of years ago. Somehow it’s survived me making many, many batches of soap since then. (That, of course, brings us to the question of “Why she was ridding herself of it in the first place?” All I can say is I’m much more of a hoarder than she is. Ha!)
As for cleaning the stainless steel bowl that I use to mix the lye with water? I carefully rinse it with a lot of water and then throw it in my dishwasher to finish up the cleaning process.

First Aid for Lye Accidents
So, you’ve decided to go ahead and make soap for the first time by following my easy, beginner soap recipe.
All is going perfectly well, until you spill the lye- and you get some on your skin.
What should you do?
Many people will say to neutralize the lye with vinegar, and that does sound like it makes a lot of sense, BUT… Think of this: Mixing a strong base with acid will set off a very strong chemical reaction.
What usually happens when you have an intense chemical reaction? Heat is given off!
So, in the effort to avoid burning yourself with a caustic substance like lye, you actually end up further burning your skin by setting off a strong chemical reaction there.
If you get lye on your skin, the first thing that you should do is rinse it off as best you can!
Run to a nearby sink and gently run fresh, clean, cool water over the area. You want to get the lye off your skin and dilute it as much as possible. Once you’ve removed it, you could consider gently cleaning the area with a mild soap, and rinsing it well.
Other lye clean-ups
If you spill lye flakes or beads?
I just pick them up by sliding them onto a piece of paper or a dustpan and then throw them into the sink. I then run cool water into the sink to flush it down.
If you spill lye solution?
I usually just wipe up the spill with a wet rag, using gloved hands, and ring it out and rinse it, as needed, throughout the clean-up process. I fully hand wash the rag with soap and water before removing my gloves and/or throwing it into the washing machine. You could also use paper towels to clean it up and toss them in the trash afterward. (In that case, I’d suggest wringing out the liquid into the sink first.)
For fun…
Other FAQ’s about lye and soapmaking:
How would you answer these now?
Over the years, my YouTube video for my beginner soap recipe has gotten a lot of questions, comments, and some bashing because of the use of lye. I understand the commenters’ thinking, but I get frustrated when people continue to argue their case without understanding what lye actually is. Hopefully, today I’ve cleared it up and you can now help defend my case in the comments section.
Would you know how to answer these now? 😉
Question 1:
“If u have to wear eye protection and gloves how can it be good for your skin? Also, I’ve only watched less than 30 seconds of it and I’ve seen a lot simpler recipes to this!”
My answer:
All soap must use lye. Soap is the reaction of the oils with the lye. The gloves and eye protection are to protect you when you first react the lye with the oils. Once the reaction has taken place, no lye remains and the soap works wonderfully with your skin. If you are afraid of working with lye, I’m afraid your only option is to use a melt and pour type soap where the reaction with lye has already taken place. Then again, you aren’t actually “making soap” anymore, you’re only rebatching the soap someone else has made and adding your extra ingredients to it. There isn’t much simpler when it comes to making soap than this recipe, but you are free to use whichever one you like.
Rebuttal comment:
“Even if it is just the initial reaction and all the lye goes away I still don’t think it is good for skin. I will try and link the simpler video I found.”
My answer:
ALL soap has lye. Use whatever video you like, but know that if you are using soap, it was made with a reaction of oils to lye. It is impossible to make soap without lye.
Comment 2:
“Lye is very poisonis for your skin”
My answer:
Lye is very caustic because it is very alkaline. It is not poisonous. That said, you are never using lye on your skin. You are using the lye to react with the oils to make soap in a process called saponification. It is impossible to make soap without lye, so if you want to avoid using anything that has ever had lye in it, even though no lye is left over, avoid all soap. You should only use surfactants, but know that many of them are harsh to the skin. You should probably also avoid most olives, soft pretzels, and some bagels as many olives are cured in lye, and many soft pretzels and bagels have been given a lye bath to give them their characteristic outside texture.
Comment 3:
“This isn’t the easy way. It’s actually the dangerous way – Lye is a Caustic acid used to clean pools and should not be used in making soap. I as a MP soaped can do the same if not more and don’t need to wait weeks for it to set to sell, I can sell mine pretty much 2hrs later and that’s using a log loaf. I use Stephenson Range that is Detergent, Palm Oil, SLS, SLES, Lye, Paraben Free bases and I sometimes add not only colour and fragrances but other ingredients like meadow foam oil, Sweet Almond Oil, Activated Charcoal, Different Assorted Mediterranean, French & Aussie Clays, Shea/Cocoa Butter, Avocado Oil, neam seed oil, jojoba Oil, Apricot Kernel Oil, Plus other assorted Fixed Carrier Oils, Coconut Oil, exfoliants, instant oats, and none of it effects the lather, just adds extra nourishment. Iv been making soap for 5 successful years now and I al make and sell the safest wax Melts and candles in the country as I use the only wax that has been dermatology tested safe for a range of body products to, I don’t make bath bombs as I find them a waste of money yet I make a Bubble bath bomb sand instead for customers to get more bang for there buck all my body products are ?% Natural & my candles won’t burn if accidentally tipped over
My answer: (Despite not understanding most of the original comment and thinking she was probably just trying to sell her products?)
If you don’t want to make soap from scratch and prefer to mix premade soap bases with other oils to personalize already made soap, that’s fine and is a good option for those who like the convenience of personalizing already made soap. If you want to actually make soap yourself, though, you need to use lye. Melt and pour soaps have already been reacted with the lye, so, yes, you can avoid working with it by buying already made soap. that said, most melt and pour soaps will have additives like propylene glycol or something that allows them to be melted into a smooth soap because natural soap doesn’t normally melt into something smooth on its own. It’s great if you have found one that you feel is natural and safe. I prefer to make my own soap, though. Lye does have a lot of great uses like curing olives and giving the characteristic crust of some pretzels and bagels.
Out of curiosity, I looked up the brand of melt and pour soaps that you mentioned, and I couldn’t find any that were actually soap-based. They appear to be surfactant blends, so they are really more of a detergent bar than a soap. Some people like to call “soap” anything in a bar form, but not all bar cleansers are actually soap-based. The first one I looked at, the goat’s milk M&P “soap” actually did use SLS and propylene glycol. They do appear to have some that use more natural surfactants, but I didn’t see any that were actually soap.
Do you still have other questions about lye?
I hope this post has helped clear things up, but if you still have questions, I’d love to hear them! I also hope you are ready to jump in and try making your own homemade soap! 🙂
 Español
Español

 Easy Zucchini Pancakes (Hobakjeon)
Easy Zucchini Pancakes (Hobakjeon)
Thai
Thanks so much for your information on lye which you have enlightened me on this.
Tracy Ariza, DDS
You’re welcome, Thai!
I’m happy it was helpful.
Lisa
Hey! So then from this, I understand that the Lush solid ‘body gels’ are rather ‘detergent bars’? How does that affect your skin?
Tracy Ariza, DDS
Some people tolerate detergents better than soap because they can be brought into a pH range that is better suited for skin and hair. It depends on the person, though.
karen
Are sodium hydroxide and potassium hydroxide interchangable?
Tracy Ariza
Hi Karen,
No, not really. They may use similar amounts, but I don’t think it’s exact.
The product you make with them is also completely different. KOH will give you a soap paste for liquid soaps while NaOH will give you a solid bar soap.
Jeff Betts
Hola,
You asked all the questions I wanted to pose to you. Thanks for a simple, yet thorough explanation of soap 101. It was fun and informative – no lye!
Tracy Ariza
Hahaha- Thanks!
Madelene
Thank you for clearing up so many of the questions I have had regarding the use of lye in soap making process and how it “disappears” once the soap is cured. Facts vs Myths!! Thanks you.
Tam
Hi, does is also apply for shampoo and conditioner? Do you need lye to make them?
Thanks in advance!
Tracy Ariza
Hi Tam,
No, you don’t need lye for either, unless you are making a soap based “shampoo,” of course (something I don’t recommend).
You can see that I don’t use lye in my homemade conditioner nor my homemade shampoo. 😉
The shampoo bar is also surfactant based and doesn’t use lye either. 😉
Ayanna Ortega
Hello! I enjoyed your blog, although I have to ask…why in every blog or article that I read about making soap without lye does no one ever talk about making soaps using saponins reach plants which where actually the first type of soap that was used by both first nations people(native America’s) and Egyptians. And they never use lye? What are your thoughts on this I wonder?
Tracy Ariza, DDS
Perhaps because the modern use of the word “soap” really refers to what you make with lye and oils. Other cleansers that work in a similar way, rather they are natural plants or are synthetic detergents, are kind of lumped together as other surfactants, but not really considered “soap” by most anymore.
Bee
Thank you for the comprehensive and insightful guide for lye and soap making. I’m in the process of gathering the ingredients.
You are incredibly patient at answering troll questions on YouTube.
Tracy Ariza
Thanks, Bee!
Yes, I try to be patient, but it is frustrating how rude some people can be there.
It’s funny because people are normally so much nicer on my blog than they are on YouTube. I’m not quite sure why.
I guess YouTube also attracts a different sort of audience. 😉
Kirsten
This is so informative and interesting, thank you!
I’ll be giving lye a go next time for sure ?
Tracy Ariza
Hi Kirsten,
I’m so glad you found it helpful and wish you the best of luck with making soap! 🙂
Penpen
Dear Tracy,
Thanks for the effort having these information shared. I use lye with my natural ingredients in making soap and it has served my purpose well. Thanks and continue the good work, God Bless
Tracy Ariza
Hi Penpen,
Thank you so much!
April C.
Hello,
Thanks for clearing that up. I would love to make a shampoo bar for myself and my daughters but I’m still scared I’ll mess up the measurements somewhere along the way.
Do you think you would ever do a recipe for a shampoo bar using a melt and pour soap? In the meantime I’ll continue to educate myself by reading your blog posts. Thanks so much for sharing so much valuable information.
Tracy Ariza
Hi April,
It’s kind of unlikely that I’ll do a soap-based shampoo post because soap-based products don’t work for my hair. The pH of soap is high. (see the pH image in the post!) It’s not ideal for most people’s hair (more ideal would be something with a pH of around 5). Some people seem to get away with using it, but it makes my hair feel like straw, and I think it would end up damaging most people’s hair with time.
I have been considering making a surfactant based shampoo bar. Those have a lower pH, making them more ideal for your hair. They still have the advantage of being waste-friendly for those who are trying to avoid packaging as much as possible.
I may also try to get a guest post from a friend who does make and use soap-based “shampoo” bars on her hair. That would give people the option to try one, even though I don’t think they’re best. That doesn’t mean other people won’t like it. 😉