Finally, a DIY hand sanitizer that is actually effective and easy to make! Learn how to make a spray or gel sanitizer.

Table of contents
With the current health scare, now is the perfect time to share a recipe that I’ve been meaning to show you for a while: how to make a homemade hand sanitizer.
Over the years, I’ve seen numerous recipes on the internet. Most of them bother me because they give a false sense of security. Just because a person has been using a homemade hand sanitizer without getting sick, doesn’t mean that it actually works.
If you’re going to go through the hassle of making a hand sanitizer (albeit very easily), why not make sure that it’s actually effective?
Possible Sanitizer Ingredients
There are many substances that can work to help sanitize. Some are practical to use, others not so much. Also, some are safer and/or easier to find than others.
Alcohol
Most commercial hand sanitizers are alcohol-based. That makes a lot of sense because alcohol kills bacteria. It’s effective against both gram-positive and gram-negative bacteria, including multidrug-resistant pathogens.
It’s not just effective against bacteria, though. Alcohol also kills yeasts and, in percentages above 60%, has been shown to reduce rotavirus, adenovirus and rhinovirus titers. For more resistant viruses like enteroviruses and Hepatitis A, a slightly higher percentage of alcohol (70-80%) may be needed.
Note
While some recipes online do use alcohol, most use concentrations way too low to actually be effective as a hand sanitizer.
Use a concentration of at least 60% for your homemade sanitizer to be effective. 70% is even better to ensure effectiveness against more types of viruses.
Chlorhexidine
Chlorhexidine is a disinfectant and antiseptic often used in clinical settings. In dental school, we occasionally prescribed chlorhexidine rinses to people with infections of teeth and gums.
While it’s often red in color, I’ve seen it sold in colorless versions which could theoretically be used to make a hand sanitizer.
Unfortunately, studies have determined that chlorhexidine alone isn’t especially effective as a hand sanitizer (by European standards, at least). a 4% solution wasn’t any more effective than soap. (Hans-P. Harke (2007), “Disinfectants”, Ullman’s Encyclopedia of Industrial Chemistry (7th ed.), Wiley, pp. 10–11)
Higher percentages may be carcinogenic, and long term use of it as a rinse isn’t recommended. That doesn’t mean that you can’t use it, of course, but alcohol is probably a more practical, effective, and safer(?) choice.
Iodine
Iodine is similar to chlorhexidine. It’s often used to disinfect in a clinical setting. While it may be used in surgical hand scrubs, it isn’t an ideal ingredient to use in a daily hand sanitizer.
Not only are iodine products normally a red color that stains, but when used at effective percentages, iodine often causes skin irritation. Not only that, but it shouldn’t be used by pregnant women (under 32 weeks) or those with thyroid problems.
There are just too many toxicity concerns to use it safely and effectively.
Triclosan
One ingredient that has been commonly used in non-alcohol hand sanitizers is triclosan. (Yes, the same triclosan I talked about in my recipe for homemade toothpaste.)
Triclosan causes several concerns, though. When combined with chlorine from water, it can form dioxins that are thought to be a carcinogen. It also poses environmental concerns as it accumulates in sewage sludge.
Even if you did want to use it (Why would you?), it’s not an ingredient that is readily available for home formulation.
Benzalkonium chloride
Another ingredient commonly found in alcohol-free hand sanitizers (perhaps the most common one), benzalkonium chloride has been associated with MRSA antibiotic resistance. It can also be irritating to the skin at concentrations greater than 0.1%.
Not only that, but, again, it isn’t an ingredient that is readily available for home use.
Essential oils
Many DIY formulations use essential oils. Some claim that they, alone, are effective at killing microbes.
While it’s true that many essential oils have antimicrobial properties, in my opinion, they aren’t potent enough to actually work their magic in a hand sanitizer.
Consider this-
Essential oils aren’t potent enough antimicrobials to be used even as preservatives in homemade cosmetics. You would need to use such high percentages to try to conserve your homemade lotions, that you’d be using concentrations that would be irritating to the skin (at best).
Compare them with alcohol. Having alcohol at 20-25% of a recipe is generally enough to preserve that product. On the other hand, 20-25% isn’t a high enough concentration for a hand sanitizer. By that, I mean that it isn’t enough to actually be effective at killing pathogens in a quick-use topical application.
So, if small amounts of antimicrobial essential oils aren’t even potent enough to use as a preservative, how are they going to be strong enough to even further and kill microbes in a hand sanitizer?
Add to that the fact that many essential oils are irritating to the skin, even at very low percentages. Some cause allergic reactions. Others cause light sensitivity. Most have to be used with caution in products made for young kids.
I’m not saying you shouldn’t use essential oils in your homemade hand sanitizer. You definitely can add them to help boost antimicrobial properties (and/or add a lovely scent).
Don’t expect to use them as the main active ingredient in your hand sanitizer, though!
Aloe gel
Aloe gel is one of the most common ingredients in homemade hand sanitizers, likely because it already has a pleasant gel-like consistency. It’s also an ingredient that is naturally soothing to the skin.
People like to use aloe gel because it’s generally considered an “all-natural” product. What most people don’t know, though, is that aloe is notoriously difficult to preserve. If you check the ingredients, many aloe gels aren’t quite as natural as you would hope.
In the interest of avoiding alcohol, people have formulated hand sanitizers with aloe gels (that often have PEG’s, parabens, and/or propylene glycol). They then mix that gel with essential oils and consider it finished. Just because it sounds “safer,” “more natural,” and “healthier” doesn’t mean that it is.
Without alcohol (or another effective active ingredient), it isn’t effective as a hand sanitizer.
Again, I’m not saying you shouldn’t use aloe gel in your formulation. What I am saying is that if “all-natural” is important to you, take a good look at the aloe gel you choose. Also, keep in mind that an effective hand sanitizer probably won’t have aloe gel as the main ingredient. It will need a high percentage of a true antimicrobial or disinfectant of some source.
You could formulate a hand sanitizer using aloe gel, but it may not be as easy as it sounds. (More about that in a minute…)
Effective ingredients for a homemade sanitizer
If you’ve read to this point, you can probably guess that the main active ingredient of my hand sanitizer is alcohol.
Non-alcohol sanitizers generally use ingredients that I prefer to avoid. They aren’t as effective as alcohol-based sanitizers. Plus, they have the added problem of being susceptible to contamination themselves.
Sure, they may be effective at killing some pathogens when used on your hands, but if they become contaminated with bacteria, they can become more harmful than helpful.
Ethyl alcohol or isopropyl alcohol
In pharmacies and the first aid section of many supermarkets, you can generally find cosmetic grade alcohol in varying percentages. For this recipe, it’s easiest to use higher-grade alcohol (like 96%), but you could use anything above the 70% range.
Here in Spain, the alcohol sold in the pharmacy section is generally ethyl alcohol. In other countries, isopropyl alcohol is more common. Either will work for this purpose, so use whichever you can find or feel more comfortable using.
This study found all types of alcohol to be effective antibacterial agents. While isopropyl alcohol was slightly more effective, it also has the drawback of having a stronger scent.
Is denatured alcohol safe to use?
When writing my posts on how to make a glycerin soap (and the vegan glycerin soap), I’ve had people comment about how methanol is added to denature some alcohol to keep people from drinking it. From what I’ve read, methanol is only added to industrial-grade denatured alcohol and not added to pharmaceutical grade alcohol.
Warning
Only use pharmaceutical grade alcohol on your skin or in your cosmetic products. Do not use industrial-grade alcohol that has been denatured with methanol.
Glycerin
Glycerin is a humectant. That means that when used in skincare products, it helps draw moisture into the skin.
Because alcohol can be drying to the skin, adding a humectant can combat that dryness and keep your skin feeling soft. Glycerin definitely improves the feel of my homemade hand sanitizer.
It may also help keep the alcohol from evaporating as quickly. While it probably only adds about a second to the drying time, every little bit may help. Prolonging the time that the alcohol is in contact with your skin is one of the main ways to boost the effectiveness of your hand sanitizer.
Essential oils
Going back to essential oils…
Yes, I like adding a couple of drops of certain essential oils to my hand sanitizer. I do it mostly to add a nice scent. If they do help boost the antimicrobial activity, though, so be it!
I sometimes use lavender, sometimes use tea tree, and sometimes use lemon oil. (If you’re worried about the phototoxicity of lemon essential oil, choose a steam distilled oil vs. an expressed oil. Or don’t use it when you’ll be out in the sun!)
If you don’t want to use essential oils, that’s perfectly fine too.
Remember: the main active ingredient is the alcohol, NOT the essential oils!
Spray vs. gel vs. foam format
Hand sanitizers are sold in several different forms. after much experimentation, I decided that a simple spray was best.
It wasn’t just to keep things easy, though…
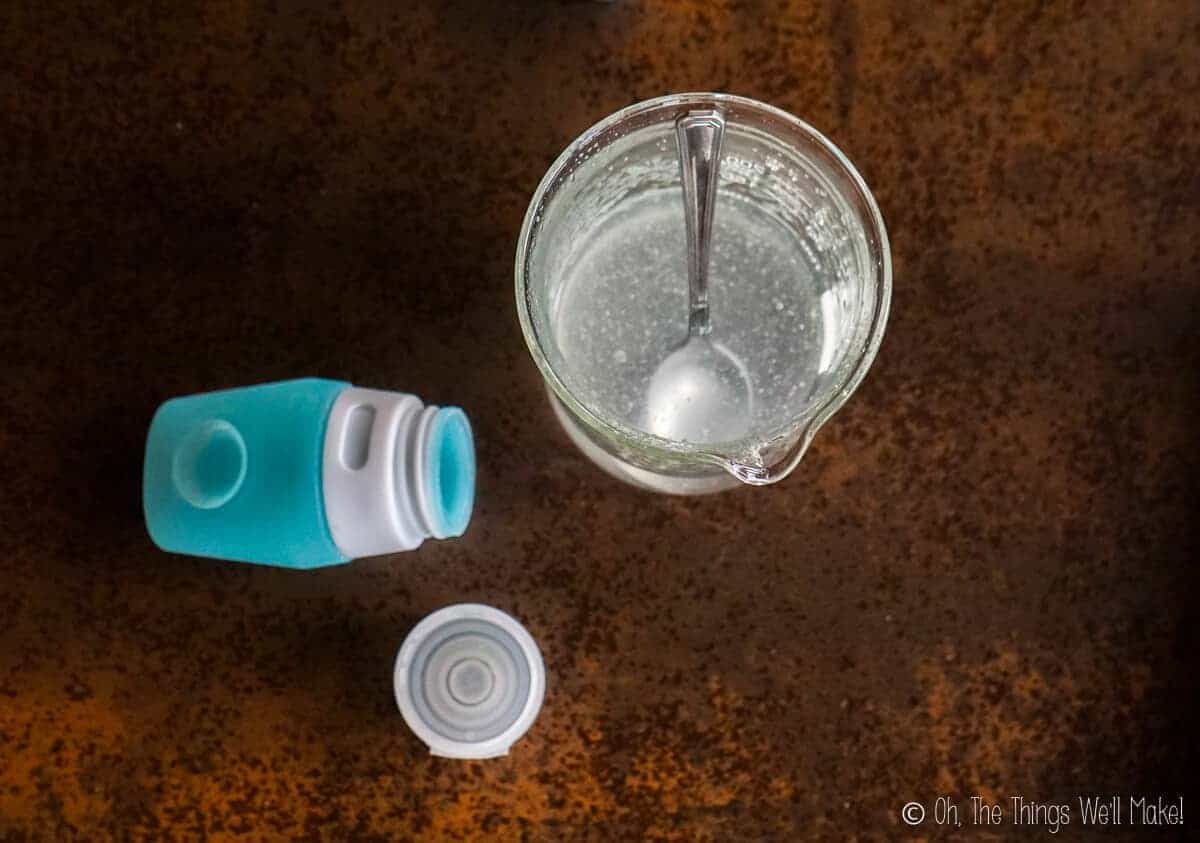
Making a gel
Because most hand sanitizers are sold in gel form, I tried to make a gel sanitizer recipe.
It seemed easy enough: Add a thickener to an alcohol mixture (of over 60% alcohol) and call it a done deal. Right?
Wrong!
Guar gum
I first tried thickening up my alcohol mixture with guar gum. To help prevent lumps in my sanitizer, I mixed the gum powder with glycerin. I added the glycerin and guar mixture to a mixture of alcohol and water.
Nothing happened.
Not only didn’t it thicken, but the gum powder settled to the bottom.
Something in the alcohol prevented the guar powder from thickening.

Guar gum settled to the bottom in alcohol 
Making a gel with water and guar gum… 
Adding alcohol turned the guar gum gel into a rubbery mess.
Just in case, I decided to change my technique. This time, I made a gel with water and guar gum. Once the gel was stable, I slowly added alcohol to that.
At first, it seemed to work. As I added more alcohol, though, the mixture separated. The guar gum became a gummy solid mess while the alcohol remained separate in a liquid form below it.
Xanthan gum
Working with xanthan gum was slightly more promising. I followed both of the above techniques for making a gel with xanthan gum instead of guar gum.
Xanthan gum seemed to incorporate the alcohol better. That was until I increased the alcohol concentration too much. Once I neared concentrations that would make an effective hand sanitizer, everything separated and fell apart.

Xanthan gum with alcohol 
Closeup of xanthan gum with alcohol showing the separation
Gelatin
Because one can successfully make Jell-o shots with gelatin and alcohol, I figured gelatin might be a more effective thickener for my gel.
Unfortunately, with the high concentration of alcohol needed for a hand sanitizer, the gelatin became a stringy, solid, rubbery mess.

Agar agar
Often used as a “vegan gelatin,” agar powder can be used to make certain types of natural gels. I even found a blogger with a recipe for an agar thickened hand sanitizer. (Interestingly enough, there was no photo or video to accompany the recipe.)
So, I felt more confident that the agar powder would work its magic and I’d finally have a natural gel sanitizer. Just to be safe, I started with the “established recipe” I found online. I boiled some water and dissolved some agar powder into the boiling water. After boiling for several minutes, I removed the mixture from the heat. As it cooled, I started to incorporate my alcohol.
While it stayed stable much longer than the gelatin, eventually the agar gel separated from the alcohol too. At lower concentrations, this may have worked. It didn’t work at the high concentrations needed for a hand sanitizer, though.
Calcium acetate
In the search for a way to make an alcohol gel, I found that some people made a DIY fuel gel using alcohol and calcium acetate.
While calcium acetate sounds exotic and hard to find, most of the tutorials began with making the calcium acetate itself.
To make calcium acetate, 1 part calcium carbonate (either the store-bought powder or from a piece of chalk) was reacted with 4 parts white vinegar (Acetic acid). Carbon dioxide is given off and the remaining liquid is supposedly a calcium acetate solution.
To make a more concentrated solution, I heated the liquid over a stove until most of it had evaporated.
I then added 1 part of the reduced liquid to 9 parts of the alcohol.
Finally, I made an alcohol gel!
The problem?
While it was a fun experiment, I didn’t end up making the type of gel I’d want for rubbing on my hands. The gel was quite solid, and some of the alcohol separated from the gel. (It was a bit like cottage cheese.)
I did play with setting some of it on fire before trying something different…
Aloe gel
After all of my unsuccessful experimentation, I decided to go back to using aloe gel. When there are so many recipes online combining aloe gel with alcohol, that must work well, right?
I figured that instead of using 2 parts aloe gel to one part alcohol (which isn’t strong enough to be effective), I’d do it the other way around. By adding 2 parts alcohol and one part aloe, I’d end up with over 60% alcohol. Yes, the consistency would be less thick and more liquidy. But, at least it would be an effective product.
Long story short- it didn’t work! As I added more alcohol, the mixture, once again, fell apart!

I ended up with a gummy, stringy mess floating in a bunch of alcohol.
In my mind, I knew that it was going to depend a lot on the thickener of the particular aloe gel used. Perhaps, this would work with an aloe gel that uses a more synthetic type thickener. It definitely isn’t going to work for all aloe gels, though. If you’re looking to make a “natural hand sanitizer,” it’s very unlikely that you’ll be able to form a gel this way.
Warning
If you plan on making a gel hand sanitizer using aloe gel, know that not all aloe gels will work. This may be possible with some but definitely won’t always work. (It’s less likely to work with the more natural brands.)
What finally worked: carbopol
While it’s not a completely natural solution, if you are set on making a sanitizer in gel format, there is one option that works.
I was just about to give up when I saw that alcohol gels could be made with carbopol. Carbopol is a water soluble polymer that works as a gelling agent. It is often used to make gels with alcohol.
That said, the place I ordered from warned about trying to make a solution with alcohol at higher than 40% of the mix. I almost didn’t order it, because I knew I needed the alcohol content to be above 60% for the gel to be effective. Because I had read elsewhere that it would work, though, I decided to buy it and give it a shot.
I figured after all I had gone through to try to make a gel for you, I’d be mad at myself if I hadn’t at least exhausted EVERY effort to get you a successful solution.
After some experimentation, I was able to make a gel. It wasn’t a thick gel, but it definitely is more viscous than the spray and it clings to your hand better and for longer. That, of course, means that the alcohol has more time to do its job of killing microbes. Perhaps adding a bit more carbopol would thicken it further. (I followed the recommended percentage dosing.)

Dissolve the carbopol in water. 
Add triethanolamine. 
Finally! A successful gel!
Making a carbopol gel
To make an alcohol gel with carbopol, you first dissolve the carbopol in the water. It takes a while to get the particles to dissolve. (Ideally, you’d want to use a magnetic stirrer and keep it stirring until the carbopol is fully dissolved and while adding the alcohol.)
Warning
Be careful not to breathe in the carbopol powder when mixing.
Once the carbopol is dissolved in the water, add the alcohol. The mixture will be very thin. That’s because we need to adjust the pH for the carbopol to work!
Add some triethanolamine to bring the pH up. You should notice that it thickens up immediately. If the carbopol wasn’t completely dissolved, it may take longer for the mixture to gel. It should thicken up further as the carbopol completely dissolves over the next hours.
Now you can add the glycerin and any chosen essential oils.
Store your gel in a silicone tube, pump dispenser, or small bottle with a flip cap top for the most convenient dispensing!
Foaming hand sanitizer
If making a gel was going to be nearly impossible, I thought I’d be clever and make an easy, foaming hand sanitizer instead.
In the past, I’ve used foaming dispensers for my homemade liquid Castile soap. I’ve also used them with my homemade micellar water to make a foaming facial cleanser.
The idea was to add a small amount of surfactant (either liquid soap or a detergent-based surfactant like the ones I talk about in my guide to natural surfactants) and dispense it with a foaming dispenser.
Normally, this works really well.
In the end, none of the surfactants I tested worked well at making foam from an alcohol mixture. The alcohol prevents the surfactants from foaming as usual.
So, while you can definitely dispense either of the hand sanitizer recipes from a foaming dispenser, it won’t really give you a foamy texture.

Make a spray
After all of my experimentation, I understood why most of the “natural companies” have chosen to make hand sanitizer sprays rather than bothering with gels or foam.
I can’t think of any natural thickener that would work effectively to make a high-concentration alcohol gel. (If you can, I’d love to hear about it!)
Dr. Bronner’s makes a hand sanitizer spray that only includes alcohol, water, glycerin, and essential oils.
Those were the main ingredients I was going to use anyway!
Why make your own?
Because all of the natural and effective hand sanitizers I’ve found are sprays and use simple ingredients, it’s really quite easy to make a similar product at home. Not only can you save yourself a lot of money, but you can choose which essential oils to use (if any).
Plus, with the mass hysteria that has hit some areas, I’ve heard that it’s actually difficult to find and buy hand sanitizers some places.
Video
Recipe

Hand Sanitizer Spray
Materials
For sanitizer spray
- 70 g 96% alcohol Use pharmaceutical grade alcohol
- 5 g glycerin
- 25 g distilled water (or a hydrosol or floral water)
- .25 g essential oils optional, a few drops, for scent
For hand sanitizer gel
- 28 g distilled water
- .3 g carbopol
- 66 g 96% alcohol
- .1 g triethylamine
- 4 g glycerin
- .25 g essential oils optional
Instructions
For sanitizer spray
- Mix together all ingredients.
- Pour into a spray bottle.
- Use, when needed, to sanitize hands by spraying over all surfaces of hands and rubbing together to completely cover them.
For hand sanitizer gel
- Weigh out the water and the carbopol. Mix them together until the carbopol is fully dissolved in the water.
- Add the alcohol and mix.
- Add the triethanolamine to raise the pH. This allows the carbopol mixture to gel.
- Add glycerin and essential oils.
- Pour the gel into a bottle with a flip-top cap, silicone travel tube, or pump bottle for easy dispensing.
Notes
How to use these hand sanitizers
To keep viruses and bacteria from making you sick, wash your hands frequently, especially after touching surfaces that have likely been contaminated by others. (That’s just about any public surface.) Frequent hand washing is still probably the best way to keep from catching a viral or bacterial infection. (Learn to make your own soap!)
That said, hand sanitizers can be used when you don’t have access to soap and water or when it’s inconvenient to wash your hands. You could consider wiping your hands with some homemade reusable wipes first when you can’t wash off visible dirt.
Hand sanitizers work best when:
- Your hands are already clean (aren’t visibly dirty).
- The sanitizer is in contact with your hands for at least 15 seconds.
- They have at least 60% alcohol.
To use, spray the sanitizer on your (clean) hands. Rub your hands together to completely cover them with the solution. If it immediately evaporates, spray again. Ideally, the solution should be in contact with your hands for at least 15 seconds.
Related posts
Natural Homemade Disinfectant Spray

Breathable 3d Face Mask

DIY Fun and Cute Face Masks

 Español
Español









 Naturally Colored Shamrock Shake (Real food)
Naturally Colored Shamrock Shake (Real food)
rajaasam
Hi, thanks for your detailed exprement , It was lot of work,
kindly find the link video https://www.youtube.com/watch?v=wa8qiBqr_4s&t=398s .
This method is similar to yours, but it came well. please try this way .
Warm Regards,
Rajaasam
Tracy Ariza, DDS
Hello,
Well, they were not able to use more than 60% alcohol because they said it wasn’t stable at higher alcohol concentrations. That’s the problem I had.
Because it’s suggested that you have more than 60% alcohol for the sanitzer to be effective, I don’t think it’s the best option. That said, it’s better than nothing.
You could definitely give it a try.
Edwin
Thanks for this piece of work.
I have been trying to make a sanitizer for my buddies but in vain. This is because my product is warerly unlike the thick ones you find in supermarkets.
I have been womdering what I’m not doing to get that thick texture and now I know. I never thought that thivkening agent would be useful here.
One question, would CMC (carboxy methylcellulose) work instead of carbapol?
Tracy Ariza, DDS
Hi Edwin,
Thank you.
Yes, that should work too. I’ve since seen it offered as an option, but it’s been selling out in my area because of the current situation, so I haven’t been able to give it a try yet. If you test it out, I’d love to hear how it goes!
Desi
Hello again Tracy 😀
I wanna ask ..
How do we know if the hand sanitizer recipe works well to kill germs? is there a simple test to prove it?
I’m worried that my homemade hand sanitizer is not effective in killing germs
Thank you ! I really hope with ur reply 😀
das menon
How about using “waterglass” [sodium silicate powder dissolved in water] as the gelling agent?
Tracy Ariza, DDS
Thanks for the tip. I can look into it when I get around to trying again.
Letitia Fendey
Suppose I add an emulsifier agent to the ethanol and calcium acetate to hold it together as an emulsion? Do you know of any? Please email me.
Tracy Ariza, DDS
Hi Letitia,
I wish I could help, but I’m not really sure. The problem is that without doing experimentation, I don’t know what will work well with such a high level of alcohol and what won’t.
sarah
Hi Tracy, there would be the possibility to teach how to make a hand soap LIQUID disinfectant with alcohol? Could it be liquid glycerin soap? I appreciate your time and teachings.
Bryan
Fantastic article…
Just wondering – if I had 85% alcohol, how do I calculate what to add of the other stuff
Tracy Ariza, DDS
Hi Bryan,
Thank you!
I found an alcohol dilution calculator that is really helpful (and takes the work out of it). You can find it here: https://www.distilling-spirits.com/tools/calculations/diluting-alcohol/?alc_c_ist=85&alc_c_soll=70&alc_v_soll=.1#result
Anyway, if you have an 85% alcohol and want a final alcohol percentage of 70%, you’d need to dilute with 20ml water+other ingredients for every 80ml alcohol for a final result of 100ml.
You decide what other ingredients you want to use-
Let’s say 4ml glycerin (which weighs about 5g,so 5% of the recipe by weight) and around 1g essential oils = 80ml alcohol plus 4ml glycerin and 1ml, more or less- probably less- of essential oils- so subtract 5 ml from the 20ml to get around 15ml of water to finish it up.
I say around because this is really depending on how much EO you use, glycerin amount, etc.- and the fact that you don’t need to hit 70% exactly.
I hope that helps.
Danny
Have you seen the one they are selling in aroma-zone? The ingredients are:
Alcohol denat, Aqua, Sodium hyaluronate, Cinnamomum camphora leaf oil, Melaleuca alternifolia leaf oil , Limonene, Linalool, Geraniol. There is no gelling agent that I can see. The only thing that could make this a gel is the HA, but they do sell it as hydroalcoholic gel, so one has to wonder. Of course it sounds expensive to waste HA, I have not felt inclined to experiment with it (and the HA I have at home is the low molecular weight, which doesn’t really gel), so we’re using spray, but I figured you might want to know.
Tracy Ariza, DDS
Hi Danny,
Wow, that IS interesting! 🙂
Yes, I agree with you about wasting HA for that. It’s an expensive ingredient for a hand sanitizer. Maybe I’ll test it out of curiosity as I’d been meaning to make more HA serum this weekend anyway. (We’re running out!)
Of course, when using the high molecular weight HA, you don’t really need much to get it to gel. So, perhaps it’s worth it for them to spend that money and make a truly natural gel.
I find the spray to be enough, too, but I’d like to see how it works so I can add it to the post for those who are set on a natural gel (if it’s successful)!
So thank you!