Knowing the pH is important when formulating cosmetics. Why, though, is it important? How should you test it? And how do you adjust it when needed?

What is pH?
To understand why pH is important, first, we need to understand pH.
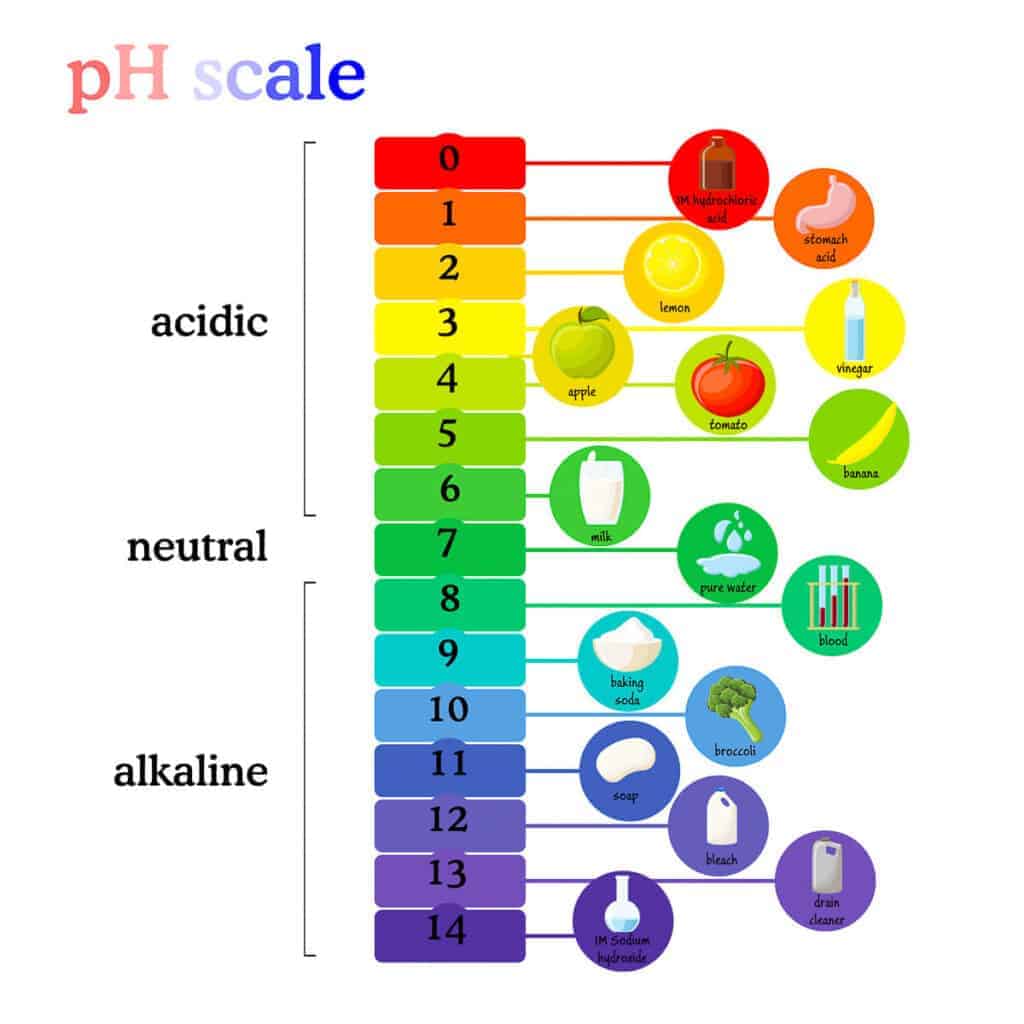
pH is a measure of an aqueous solution. It tells us about the acidity or alkalinity of the solution on a scale from 0 to 14. In general terms, neutral substances measure 7 on the scale (or 6.6-7.3). Because pH is actually a measure of hydrogen ions in a solution, the pH of a substance is somewhat temperature-dependent. (More hydrogen ions are released at higher temperatures.) In simple terms, though, pure water such as distilled water will have a neutral measurement of 7.
Acidic solutions, those with more free hydrogen ions, will have a pH reading of less than 7 while alkaline (aka. basic) substances have a pH of over 7.
Strong acids will have smaller numbers and anything with a pH of under 3.5 is considered ultra-acidic. Solutions with the highest pHs are the most alkaline.
Both solutions that are either extremely acidic or extremely alkaline are very caustic and can burn your skin.
pH of skin
While pH is really a measure of water-based solutions, you will likely hear people talk about the pH of our skin. They’ll say that our skin is acidic.
It’s not really that our skin is acidic, but It is covered with secretions like sweat mixed with bacteria and oil. These secretions and other substances are on the acidic side and protect our skin. They’re called the acid mantle. When you disrupt or somehow remove the acid mantle, your body works quickly to replenish and restore it.
Babies are born with a more neutral skin pH. As they age, they form more of a protective acid mantle, and the pH of their skin lowers. Different areas of our body can have different pH values too. More exposed areas generally have a higher pH than the more protected hidden areas like armpits or genital areas whose protective acid mantle stays more intact.
Most people are able to recover from changes in pH of the skin without issues. That’s why you can use natural soap, which is on the alkaline side, and not necessarily see any negative effects. Some people, though, find those changes in pH to be too much for their sensitive skin. In those cases, it’s generally best to use any cleansers sparingly. When a cleansing agent is needed, though, people with sensitive skin may benefit from using a gentle, non-soap cleanser with a lower pH.
Ideal pH for skin products
Generally, a pH of 5-6 is a good range to shoot for when making cleansers and lotions for the skin.
pH of Hair
While skin generally recovers quickly from changes in pH, hair isn’t usually as resilient. Apart from the follicle, hair is mostly dead. It can’t repair and protect itself in the same way that skin does.
If you’ve followed my blog for a while, you probably know that I love making soap. Despite that love of soap, though, I have not and will not use it in my hair. The high pH of soap damages hair in several ways.
High pH damages the hair cuticle
Just as skin has a protective layer (the acid mantle), hair also has a protective layer called the cuticle. The cuticle, when seen under a microscope, is made up of many scales that overlap. When in place and properly overlapping, they keep moisture in the hair shaft. Flat cuticle scales protect the hair more and give a smooth, shiny appearance to the hair.
When you use alkaline products on your hair, the cuticle scales lift up and open. This leaves your hair prone to dryness and breakage. Hair with raised cuticle scales is more likely to tangle and break and also look frizzy.
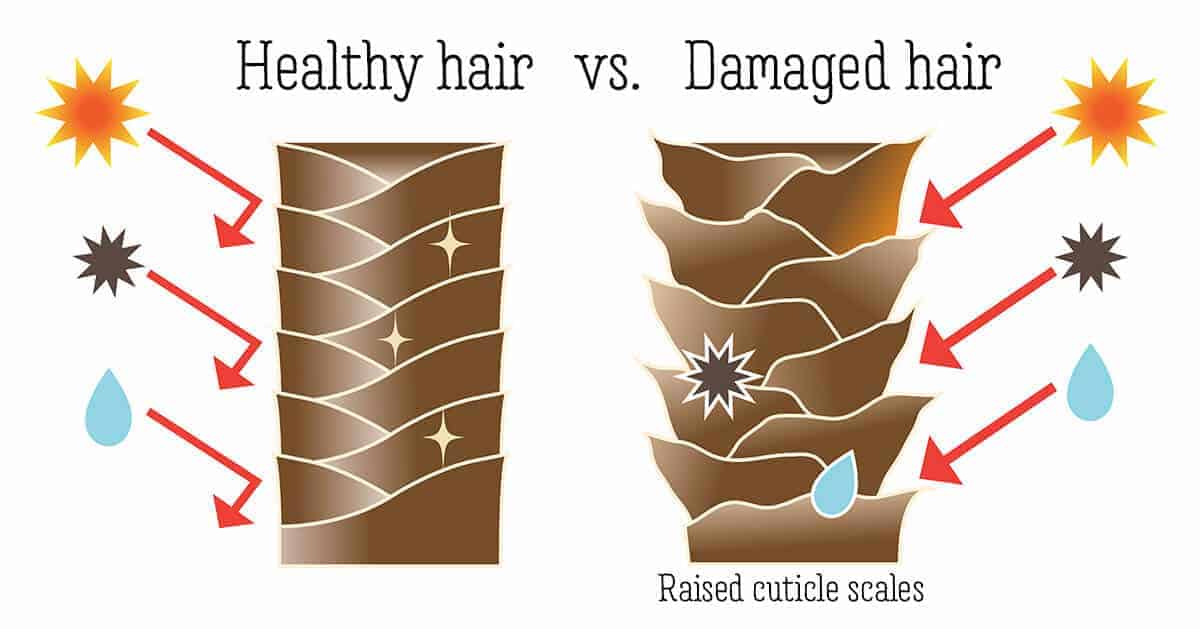
In an attempt to fix that problem, sellers of soap-based “shampoo bars” will tell you to do a final rinse with vinegar to lower the pH again. That can temporarily help with the appearance of the hair, lowering the scales of the cuticle, allowing it to shine again. Over time, though, the constant opening and closing of the cuticle generally leaves hair damaged. Someone with short hair, or someone who doesn’t wash their hair with soap very often, may not notice the ill effects. Constantly washing long hair with soap, though, is almost sure to eventually damage it beyond repair.
Other ways soap can damage hair
Not only can the alkaline pH of soap damage the cuticle layer, but it can also increase the negative charge of the hair which leads to increased friction between hair strands. The friction can result in more breakage of the hair.
Apart from that, soap can leave an alkaline residue on the hair that precipitates in the form of calcium salts. These salts build up on the hair, making hair look dull and, again, more prone to tangling and breaking.
Ideal pH for hair products
Ideally, most shampoos will fall in a pH range of 4.5-5.5. Children’s shampoos tend to have a slightly higher pH, mainly because the closer the shampoo is to a neutral pH of 7, the more likely it will be “tear-free” if it gets in the eyes. I talk more about that in my baby wash and shampoo recipe post.
pH and cosmetic ingredients
Obviously, then, the pH of a cosmetic will determine how it will affect your skin or hair. That isn’t the only thing to consider, though. The pH of a cosmetic will also affect how its ingredients will behave.
pH and preservatives
If you are making a product with water in it, you’ll almost always need to add a preservative to keep unwanted bacteria, molds, and other microbes from developing in it. Microbial growth can lead to skin irritation and infections. While mold can generally be seen, bacterial growth is often invisible to the eye.
Not all preservatives work at all pHs, though. In fact, I don’t know of any preservative sold for home use that is effective at all pH levels. When adding a preservative, you’ll need to check the effective pH range in which your chosen preservative is effective. (Your supplier should give you that information.) Then, you’ll need to check your homemade product to make sure it falls into that range.
Generally, products with extreme pHs are self-preserving because most pathogenic microbes won’t happily grow in an extreme pH environment. In most cases, though, you’ll want to make products that fall into a more neutral (yet slightly acidic) range. So, you’ll need to add a preservative. (For more information, check out my beginner’s guide to natural preservatives.)

pH effects on surfactants, emulsifiers, and other ingredients
The pH of a product may also affect its other ingredients. It could affect the stability and/or effectiveness of certain emulsifiers, surfactants, and/or other ingredients. It may also affect the viscosity of certain gums and other thickeners.
In my tutorial for how to make a hand sanitizer gel, I used TEA (triethanolamine) to raise the pH of my mixture in order for it to gel properly.
Amphoteric surfactants have both a positive and negative charge in the same molecule. Some will actually change their properties based on the pH, changing their charge from positive to negative as the pH of formula increases. So, they may be cationic at a low pH and anionic at a high pH, and be “zwitterionic” in the middle. (I discuss the different types of surfactants in my guide to natural surfactants.)
While most cosmetic ingredients will be fine to use in the range that is generally desirable for most skin and hair products, it’s a good idea to check on the pH recommendations for any new ingredients you plan on using. That’s especially important if you plan on changing their pH drastically for some reason or another.
How to test pH
There are several ways to test the pH of a product.
pH test strips
The easiest and cheapest way to check pH is to use simple paper test strips. pH test strips will change color when dipped into an aqueous solution. The color can be compared to a color key that comes with them in order to determine, more or less, what the pH is.
There are a variety of types of test strips available. Some will show a wide range of pH values relatively well, while others will focus on a particular pH range and give more variances of color within that range to give a more precise measurement.
Because it is often difficult to distinguish certain colors when comparing to the color key, pH strips aren’t normally accurate when you need to know a precise pH. At certain pH ranges, you may be able to tell that your reading is somewhere in a range between 2 or 3 numbers, but you wouldn’t be able to say for certain that the pH was accurate to a decimal place reading.
When formulating cosmetics at home, pH strips will normally be accurate enough to show you whether or not you have made a product in the range you need. In. In most cases, you won’t need to know that your shower gel has a pH of exactly 5.4. Just knowing that you are in a skin and hair-friendly range of between 4-5 is probably enough.

Kids generally love playing scientist with pH test strips. It’s a fun activity for them to learn more about acidity and alkalinity. Similarly, my son was absolutely amazed to find that certain foods will also magically change color depending on their pH. I made magical, color changing homemade food coloring using those foods!
pH meters
Some people may want more precision, though. They may find investing in some sort of pH meter worthwhile.
While pH meters are more precise, they are also more expensive and generally have upkeep that needs to be done to keep them working properly. They normally need to be calibrated to make accurate readings. They will also generally have a longer lifespan and will work better when stored wet. If the electrodes completely dry out, the pH readings can lose their accuracy.
If you aren’t using it often, and don’t need precise measurements, it’s probably not worth spending the money on a pH meter. (I’d invest in a good kitchen scale (for bigger recipes) and a jeweler scale (for smaller recipes) instead!)
Testing the pH of non-aqueous products
There are times that you may want to know the pH of a solid product, or one that doesn’t have water in it. This is generally important for products that will be used on the hair or skin in the presence of water like a shampoo bar or a bar of soap.
To read the pH of these non-aqueous products, you’ll need to somehow mix them with water. To know their true pH, it’s best to mix them with distilled water. If you are making the products for yourself and you concerned about the pH of the product when it is being used at your home, you can mix it with your tap water.
So, for example, let’s say you want to test the pH of a homemade shampoo bar. Take some water and lather up the shampoo bar. Then, use a test strip to take a reading of the resulting lather.
Distilled water will have a neutral reading of around 7. Home tap water generally has minerals and other substances that can affect the pH, meaning it may be more acidic or more alkaline than distilled water. If you lather it up with your tap water, it give give your an idea of the pH of your product when you are using it at home.

Adjusting the pH
There are times when you may need to adjust the pH of a product. For liquid products, it’s normally pretty simple. Just work slowly, adding pH adjusters as needed, so that you don’t find yourself having to go up and down again.
For shampoo bars and conditioner bars, you’ll have to test the pH once the bars have set. If you need to adjust the pH, you’ll have to do some guesswork, adding a pH adjuster to the next batch and testing the final pH to see if you have guessed correctly. In these cases, it’s especially important to keep track of how much of the pH adjuster you add each time, so that you can easily reproduce a successful recipe time after time.
Lowering the pH
If the pH of your product is too high, you’ll need to adjust the pH down with an acid of some sort. Acids that are commonly used are citric acid or lactic acid.
Lactic acid is generally sold at cosmetic supply places in strong solutions. In most cases, for small batches like the ones I share here, you’ll only need to add a drop or two to lower the pH of whatever product you are making.
Citric acid is often sold in powder or crystal form. To use it, make a solution by dissolving the citric acid in water. I like to make a 10% solution by using 1g citric acid for every 9g of distilled water. By measuring how much you add, and using a set solution, you can easily reproduce your recipe each time.

Lowering the pH of soap
Some people try to lower the pH of soap, especially when making soap-based “shampoo” bars, by mixing it with acidic ingredients. The problem with doing so is that by lowering the pH of soap too much, it will eventually fall apart and won’t work like soap anymore.
Soap can never be acidic or even neutral. I discuss the problems of trying to change the pH of soap in my post about “neutralizing” liquid soaps.
Raising the pH
While it’s less common, there are times when you may need to raise the pH.
This can be done with solutions of sodium hydroxide (NaOH) or potassium hydroxide (KOH). Soap makers will generally have both of those types of lye on hand for making their soaps. Be careful when mixing the lye solutions as mixing lye with water causes a chemical reaction that gives off heat and vapor that you should avoid inhaling. (Wear safety goggles and gloves when mixing the solution!)

Some people try using baking soda to raise the pH, but you’ll need much more baking soda to effectively raise pH.
TEA (triethanolamine) and arginine are also commonly used to raise the pH of certain substances. I used TEA to raise the pH when working carbopol to make the hand sanitizing gel.
When working with specialized ingredients like Emulsense HC, a natural cationic emulsifier that can be used to make hair conditioner, they suggest preserving it with Spectrastat G2-N, a preservative that is most effective at a pH of between 5-5.5. They suggest raising the pH with arginine since the other common pH increasers like NaOH and baking soda don’t work with their system.
Other posts that discuss pH
For more information about pH and for a fun project that kids will love, check out these posts…

Free Formula Botanic Masterclass!
Running now…
9 free 10-minute classes to help you learn to begin developing professional-grade natural cosmetics in your own home!
 Español
Español





 Chocolate Dates with Almonds
Chocolate Dates with Almonds
ანა
ძალიან დიდი მადლობა . მეტად საჭირო ინფორმაცია იყო.
Tracy Ariza, DDS
You’re welcome!